2020 Volume 45 Issue 3 Pages 131-136
2020 Volume 45 Issue 3 Pages 131-136
Reproductive disorders in birds are the most characteristic effects of DDT contamination of wildlife. Experimental exposure of avian eggs to the estrogenic substance o,p′-DDT causes abnormal development of the reproductive tract (shortening of the left oviduct and aberrant development of the right oviduct) and eggshell thinning in mature birds, but it is still not known how eggshell thinning occurs in the abnormal oviduct. To fill this information gap, we examined the histology of the uterine part of the oviduct in Japanese quail treated in ovo with o,p′-DDT or a synthetic estrogen, diethylstilbestrol (DES), and we performed immunohistochemical staining for the calcium-binding proteins CALB1, SPP1, and TRPV6. Both o,p′-DDT-treated and DES-treated quail had few, and scattered, gland cells in the left uterus, unlike vehicle controls, in which gland cells tightly occupied the lamina propria. The aberrantly developed right uterus retained all the components of the normal left uterus, but in immature form. Immunostaining for CALB1, SPP1, and TRPV6 was greatly reduced by both o,p′-DDT and DES; SPP1 and TRPV6 immunostaining patterns, in particular, differed distinctly from those in the controls. These findings suggest that CALB1, SPP1, and TRPV6 are molecular factors, decreased production of which is responsible for eggshell thinning. Our findings also could contribute to understanding of the eggshell formation mechanism in birds.
From the 1950s to the 1970s, reproductive disorders were observed in wild raptorial and fish-eating birds in organochlorine-compound-contaminated areas of developed countries. o,p′-Dichlorodiphenyltrichloroethane (DDT), an estrogenic and minor component of commercial DDT, is regarded as the main causative agent. Experimental exposure of embryos to o,p′-DDT or synthetic estrogens causes abnormal development of the reproductive tract (shortening of the left oviduct and development of the usually rudimentary or absent right oviduct) and resultant reduction of eggshell-forming ability in poultry (Halldin et al., 2003; Holm et al., 2006; Kamata et al., 2009b). We reported previously that female Japanese quail treated in ovo with an estrogenic compound, diethylstilbestrol (DES), also possessed abnormally developed oviducts and laid thin-shelled or shell-less eggs at maturity. The eggshell thinning occurred particularly in two of three layers—the mammillary and cuticular layers—of the eggshell (Kamata et al., 2009a). In the uterine part of the abnormally shortened left oviducts there is a significant decrease in the mRNA transcripts of the genes encoding the calcium-binding proteins calbindin-D28k (CALB1) and osteopontin (SPP1) compared with in normal controls. In the normal eggshell, SPP1 is localized to the layers where we found marked eggshell thinning (Fernandez et al., 2003). These findings indicate that calcium mobilization for eggshell formation is disrupted in the uteri of birds exposed to o,p′-DDT or xenoestrogens. However, we have a poor understanding of how the factors involved in calcium mobilization act in eggshell formation and of how changes in these factors are responsible for eggshell thinning. Therefore, our aim here was to obtain information about the molecular mechanisms of eggshell thinning. In our previous studies we had not histologically investigated the abnormally developed oviducts, so here we studied the microscopic appearance of the uteri of quail treated in ovo with o,p′-DDT or DES. To ascertain the mechanism of eggshell thinning, we immunohistochemically determined the production levels and distributions of the calcium-binding proteins CALB1, SPP1, and transient receptor potential cation channel, subfamily V, member 6 (TRPV6), in the uterus.
Experiments were performed with the approval of the Animal Ethics Committee of the National Institute for Environmental Studies, Tsukuba, Japan.
The study used the same oviduct samples we had prepared from Japanese quail (Coturnix japonica) treated in ovo with the test compounds in our previous study (Kamata et al., 2009b). Briefly, eggs were collected from white egg (WE) female and Brazilian brown (Br) male pairs. To minimize the differences between the average egg mass in the groups, 35 eggs were randomly selected for each group. Test solution (20 μL) of o,p′-DDT (Accu Standard, Inc., New Haven, CT, USA) or DES (Sigma Chemicals, St. Louis, MO, USA) dissolved in corn oil (containing 1% dimethylsulfoxide [DMSO] as a solubilizing agent) was injected into the yolk. Thirty successfully injected eggs were incubated at 37.8°C and 60% relative humidity, with a turning cycle of once an hour. After hatching, seven female and seven male chicks, without apparent malformations, were randomly selected from each treatment group and raised in mixed-sex groups. Quail at 5 weeks of age were individually moved to single stainless-steel cages, and the eggs laid by each female quail were collected. Female quail were sacrificed at 10 weeks of age and necropsied for examination of reproductive system morphology. In our original study (Kamata et al., 2009b) the quail were treated in ovo with o,p′-DDT at doses of 0 (vehicle alone, 1% DMSO in corn oil), 1, 10, 30, or 100 μg/g of egg, or with DES at 1 or 10 ng/g of egg. Here, for histological analysis, we used the uterine parts from those females in our previous study that had been treated in ovo with 0, 1, or 100 μg o,p′-DDT/g or 10 ng DES/g. The doses of o,p′-DDT were selected as presumable levels in the eggs of wild birds living in highly polluted regions during the 1960s and 1970s, because several hundred ppm of DDT homologs were reported in the eggs of wild birds at that time (Gilbertson and Fox, 1977; Lamont et al., 1970).
Histopathology and immunohistochemistryCollected uteri were immersed in 10% formalin solution, dehydrated, and embedded in paraffin by using routine methods. The paraffin blocks were cut into thin sections (4 μm) and stained with hematoxylin and eosin. Histopathological changes were observed under a light microscope.
Sections of the uterus were also immunostained for CALB1, SPP1, and TRPV6. The primary antibodies Polyclonal Rabbit Anti-Calbindin-D-28K (KD-15) (C7354, Sigma-Aldrich, St. Louis, MO, USA), Polyclonal Rabbit Anti-OPN (AP11567a, Abgent, San Diego, CA, USA), and Polyclonal Rabbit Anti-TRPV6 (ACC-036, Alomone Labs, Jerusalem, Israel), were used at concentrations of 1:500, 1:100, and 1:200, respectively. Because Japanese quail are not commonly used as experimental animals, we could not obtain species-specific target proteins and could not examine the reactivities of these primary antibodies in quail. However, the cross-reactivity of the CALB1 antibody against its target protein in chickens has been confirmed by its manufacturer, and immunostaining of chicken tissues with TRPV6 antibody has been reported previously (Yang et al., 2013). We found here that the CALB1, SPP1, and TRPV6 antibodies immunostained specific regions of the quail uterus; as described in the DISCUSSION, the respective proteins are located similarly in chickens. We therefore considered these three antibodies to be cross-reactive against the quail proteins. Immunostaining was performed with N-Histofine Simple Stain MAX PO (R) (Nichirei, Tokyo, Japan) in accordance with the manufacturer’s instructions. Immunoreactions were visualized with diaminobenzidine solution (Nichirei) followed by counterstaining with Mayer’s hematoxylin. To evaluate protein production levels, micrographs of 10 areas per section at random were recorded by using a digital microscope (KH-8700, Hirox, Tokyo, Japan), and the proportion of specifically stained areas in the total area of each recorded section was calculated by using the RGB extraction tool of dedicated software (Hirox).
StatisticsStatistical analyses were performed with SigmaStat for Windows version 3.1.1 (Systat Software, Inc., Richmond, CA, USA). Differences between control and treatment groups were tested by using Dunnett’s multiple comparison test. Because collected uteri were chosen randomly and were used not only for histology but also for our previously reported analyses (Kamata et al., 2009b), the number of samples used in this study was three to six per group; the exception was that because of a low rate of occurrence of the well-developed (not rudimentary) right oviduct we used only two right uteri from females treated with 100 μg o,p′-DDT/g. Results are expressed as means ± SEM, and statistical significance was noted when P < 0.05.
This study used oviduct samples from the same individual quail as prepared in our earlier study (Kamata et al., 2009b). In this paragraph, to better explain the effects of o,p′-DDT in relation to our current results, we present excerpts from that previous study. We previously reported the effects of in ovo treatment with o,p′-DDT and DES on development and reproduction in Japanese quail in terms of such factors as hatchability of treated eggs, growth of hatched chicks, egg production by females, and development of the reproductive organs. Our previous findings with regard to development of the reproductive tract and egg production and eggshell formation capacity are particularly relevant to our current study: compared with the results in the controls, the total number of eggs laid by female quail during the experimental period was significantly decreased at a dose of 10 ng DES/g of egg, and eggshell quality (eggshell strength, weight, and thickness) was decreased both by o,p′-DDT (10 to 100 μg/g) and DES (1 and 10 ng/g). In ovo treatment with o,p′-DDT or DES dose-dependently shortened the left oviduct in female quail aged 10 weeks. The right oviduct was unusually developed in two or six of seven female quail at high doses of o,p′-DDT (100 μg/g) or DES (10 ng/g), respectively: in normal female birds only the left—not the right—reproductive organ develops. The right oviduct was much shorter than a normal left oviduct but had a visible oviduct structure.
Uterine histopathology and immunohistochemistryThe oviduct uterus or shell gland—the site of eggshell calcification and pigmentation—was examined microscopically. In uteri from control female quail, the simple columnar or partly stratified epithelium, lamina propria, two smooth muscle layers, and serosa were observed. Gland cells occupied the lamina propria so tightly that the ductal structures of the gland cells were barely identifiable (Fig. 1a). In females treated in ovo with 100 μg o,p′-DDT/g, the lamina propria of the left uterus was clearly thinner than that in the controls, and the gland cells retained a clear but sparse ductal structure (Fig. 1b). Seemingly all components of the normal uterus were observed in the aberrant right uteri of females treated with 100 μg o,p′-DDT/g. However, their tissue architecture was immature, gland cells in the lamina propria were very sparse, and there were few glandular ducts (Fig. 1d). One microgram of o,p′-DDT/g had barely detectable effects on the uterus, which appeared broadly similar to that of the controls (data not shown). DES (10 ng/g) also impaired left uterine development and induced the development of the right uterus; similar to the results at a high dose of o,p′-DDT, glandular ducts were clear but sparse in the lamina propria of the left uterus, and the tissue architecture of the right uterus was immature (Fig. 1c and 1d).

Representative histological changes in oviduct uteri from Japanese quail. Left uteri from quail treated in ovo with vehicle only (a), 100 μg o,p′-DDT/g (b), or 10 ng DES/g (c). (d) Right uteri from quail treated with o,p′-DDT (left) or DES (right). Bars = 100 μm.
Immunostaining of the uterus of control quail with CALB1 antibody was found in the entire cytoplasm throughout the gland cells and on the luminal sides of the epithelial cells (Fig. 2a). In ovo treatment with o,p′-DDT dose-dependently decreased CALB1 immunostaining in both the gland cells and the epithelium of the left uterus; the average immunostained area in the group treated in ovo with 100 μg o,p′-DDT/g was significantly smaller than that in the controls (Fig. 2b and 3a). DES (10 ng/g) also significantly decreased CALB1 immunostaining in the left uterus compared with that in the controls (Fig. 2c and 3a). The CALB1-immunostained areas of the right uterus of females treated in ovo with 100 μg o,p′-DDT/g or 10 ng DES/g were significantly smaller than that of the left uterus of the controls and were respectively comparable to those of the corresponding left uteri (Fig. 2d and 3a). In control quail, immunostaining of the uterus for SPP1 protein was also observed in the entire cytoplasm throughout the gland cells and on the luminal sides of the epithelial cells, and strong SPP1 immunostaining occurred in the apical regions of the epithelium (Fig. 4a). o,p′-DDT or DES administration in ovo at all tested doses greatly and significantly decreased SPP1 production in the left uterus (Fig. 3b), but SPP1 immunostaining remained strong in the luminal membranes of the ductal gland cells (Fig. 4b and 4c). The SPP1-immunostained area of the right uterus was very small in both o,p′-DDT-treated and DES-treated females compared with that of the left uterus in the controls (Fig. 3b). TRPV6 immunostaining in the uterus of control females was very weak but was observed throughout the entire cytoplasm of the gland cells (Fig. 5a). Strong TRPV6 immunostaining occurred on the luminal sides of the epithelial cells, especially in their apical regions. At all tested doses, o,p′-DDT greatly, and DES moderately, decreased TRPV6 protein production in the left uterus (Fig. 3c), but TRPV6 immunostaining remained strong in the cytoplasm on the luminal sides of the ductal gland cells (Fig. 5b and 5c). The TRPV6-immunostained area of the right uterus was extremely low in both o,p′-DDT-treated and DES-treated females compared with that of the left uterus in the controls (Fig. 3c).

Representative images of oviduct uteri stained immunohistochemically for CALB1. Left uteri from Japanese quail treated in ovo with vehicle only (a), 100 μg o,p′-DDT/g (b), or 10 ng DES/g (c). (d) Right uteri from quail treated with o,p′-DDT (left) or DES (right). Bars = 100 μm.

Quantification of immunohistochemically stained areas in oviduct uteri. Y-axis values represent the percentage of areas stained specifically for CALB1 (a), SPP1 (b), and TRPV6 (c) in the total area of each section. X-axis values represent distinctions of left or right uterus and of treatments: Cont, DDT1, DDT100, and DES, respectively, are left uteri from quail treated in ovo with vehicle only (control), 1 μg o,p′-DDT/g, 100 μg o,p′-DDT/g, or 10 ng DES/g; DDT100R and DESR, respectively, are right uteri from quail treated with 100 μg o,p′-DDT/g or 10 ng DES/g. Asterisks indicate levels of significance of differences from the vehicle controls: *P < 0.05, **P < 0.01, ***P < 0.001. # Only two right uteri were tested because of low rate of occurrence of a right oviduct.

Representative images of oviduct uteri stained immunohistochemically for SPP1. Left uteri from Japanese quail treated in ovo with vehicle only (a), 100 μg o,p′-DDT/g (b), or 10 ng DES/g (c). Bars = 100 μm.
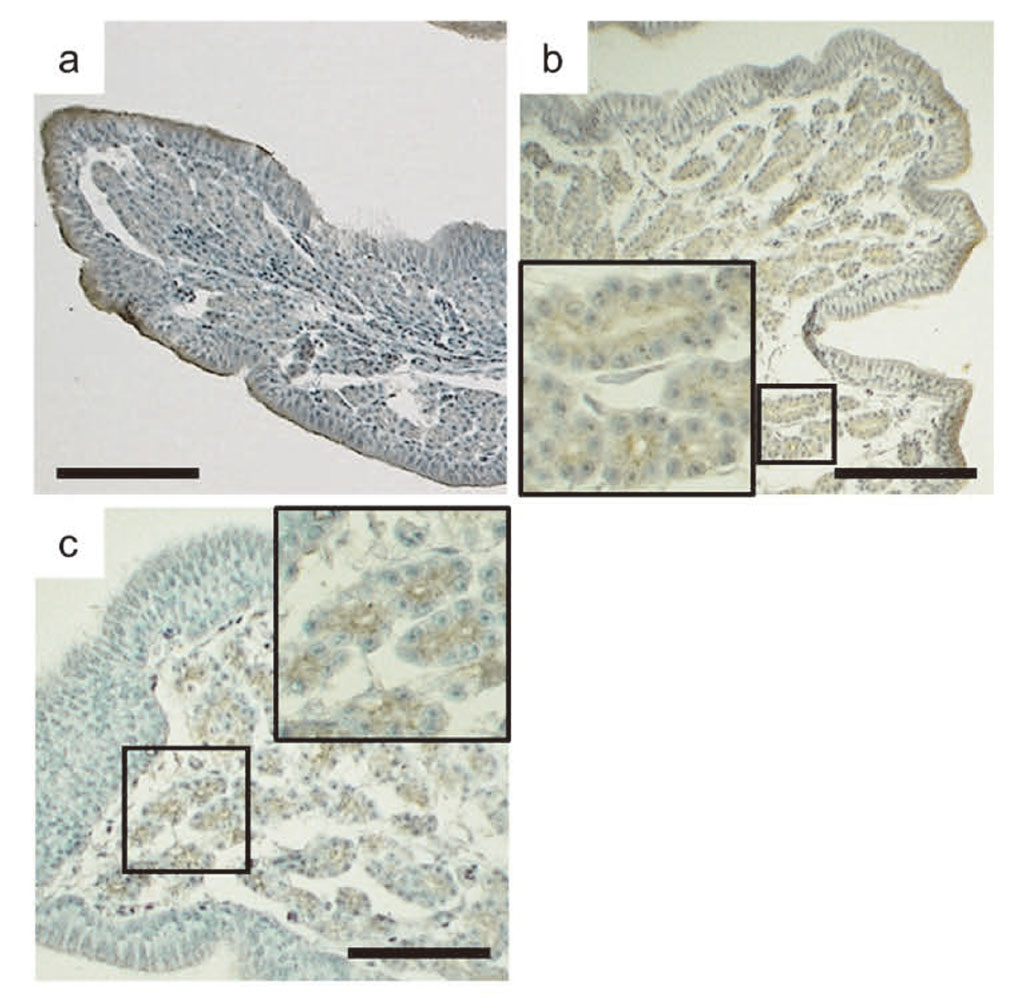
Representative images of oviduct uteri stained immunohistochemically for TRPV6. Left uteri from Japanese quail treated in ovo with vehicle only (a), 100 μg o,p′-DDT/g (b), or 10 ng DES/g (c). Bars = 100 μm.
Because abnormal growth of the reproductive tract and eggshell thinning in birds are the most characteristic effects of embryonic exposure to estrogenic compounds, it is not surprising that uterine structure was deficient in both o,p′-DDT-treated and DES-treated female quail. Similar to our observations, Halldin et al. (2003) reported that female quail treated embryonically with o,p′-DDT (150 μg/g of egg) on day 3 of incubation had few and scattered gland cells in the uterus, unlike vehicle controls, in which gland cells tightly occupied the lamina propria. Aberrant estrogenic action on the embryos suppressed the growth of the left uterus. In addition, we found that the aberrantly developed right uterus had all of the components—but immature ones—of the normal left uterus. Interestingly, embryonic xenoestrogens induce the growth of the right reproductive tract, as opposed to the left. Although the morphological changes of the avian reproductive tract in response to embryonic xenoestrogens have been fairly well documented (Halldin et al., 2003; Holm et al., 2006; Kamata et al., 2009b), the details of how eggshell thinning occurs in the abnormally developed oviduct remain unclear.
Our observed defective development of the left oviduct and reduced production of the three calcium-binding proteins CALB1, SPP1, and TRPV6 in the uterus coincided closely with eggshell thinning in quail treated in ovo with o,p′-DDT or DES (Kamata et al., 2009a, 2009b). Production of molecules associated with calcium mobilization, such as these calcium-binding proteins, has been examined to further our understanding of their role in eggshell formation. In mammals, CALB1 (or calbindin-D9k, S100G) and TRPV6 (or TRPV5) are produced mainly in the intestine, kidney, uterus, and placenta and play crucial roles in transcellular Ca2+ transport; in the intestine, following passive entry of Ca2+ into enterocytes through capture by TRPV6, Ca2+ bound to CALB1 diffuses into the cytosol to the basolateral membrane, and Ca2+ is extruded via Ca2+-ATPase or Na+-Ca2+ exchanger, or both (van Abel et al., 2005). These two proteins have been observed in the oviduct of birds, but their functional properties are poorly understood. CALB1 is present in the cytosol of gland cells of the uterus at high concentration (Jande et al., 1981), and strong TRPV6 immunostaining is found specifically in the apical regions of the luminal epithelium of the uterus (Yang et al., 2013). These findings generally agreed with our results, but we also observed CALB1 in the normal uterine epithelium and TRPV6 throughout the cytoplasm of the gland cells. SPP1 is regarded as a multifunctional protein and is produced in various tissues. From findings such as its tissue distribution, its affinity for calcium, and its content in bone and teeth, SPP1 is thought to play a particularly important role in bone and teeth mineralization and is therefore also suggested to be important for eggshell mineralization in birds (Pines et al., 1995). SPP1 and its mRNA have been reported to be exclusively located in the epithelial cell layer of the uterus (Pines et al., 1995), unlike our finding that SPP1 was present both throughout the normal gland cell cytoplasm and in the epithelium. Because production of CALB1, SPP1, and TRPV6, or expression of their genes, or both, has been observed or found to be elevated during eggshell calcification, when the egg is present within the uterus (Jonchère et al., 2012; Pines et al., 1995; Yang et al., 2013), there can be little doubt that these proteins play key roles in eggshell calcification. Hypoplasia of the uterus caused by o,p′-DDT or DES leads to depletion of molecules involved in eggshell calcification, such as the calcium-binding proteins examined here, and could thus impair eggshell-forming ability. Interestingly, we found strong immunostaining for SPP1 and TRPV6 specifically on the luminal sides of the ductal gland cells in hypoplastic uteri, unlike in normal ones. These two proteins may be produced in increased amounts in this region to act in calcium transport to compensate for the calcium insufficiency attributable to uterine hypoplasia. In addition, SPP1 and TRPV6 were located in the luminal sides of epithelial cells as well as throughout the cytoplasm of the gland cells, suggesting that these proteins (especially TRPV6) act to excrete calcium into the uterine lumen in a way differently from in the intestine, in which TRPV6 is solely responsible for Ca2+ entry into enterocytes. Our findings suggest that decreased production of the molecular factors CALB1, SPP1, and TRPV6 is responsible for the eggshell thinning caused by embryonic exposure to xenoestrogens. These results could contribute to our understanding of the still largely unknown mechanisms of eggshell formation in birds.
We thank Dr. Shinji Takahashi, Mr. Akira Shimizu, Ms. Akane Hirokawa, Ms. Marika Fukui, Mr. Syunsuke Sasaki, Mr. Keito Yunokawa, Ms. Yoko Okada, Ms. Miho Yamasaki, and Mr. Toshiaki Ito for their skillful technical help and useful discussions. This work was funded in part by the Ministry of the Environment, Japan.
Conflict of interestThe authors declare that there is no conflict of interest.